Element no 10 crossword clue – Embark on an exciting journey to uncover the secrets behind element no. 10, a captivating element that holds a unique place in the periodic table. From its discovery to its remarkable properties and applications, prepare to be amazed as we delve into the fascinating world of element no.
10.
Element no. 10, also known by its chemical symbol Ne, is a noble gas that occupies a special position in Group 18 of the periodic table. Its atomic number, 10, signifies its 10 protons and 10 electrons, while its atomic weight of 20.18 atomic mass units reflects its mass.
Element No. 10 in the Periodic Table
In the vast expanse of the periodic table, element number 10 stands out as a significant player. It is a noble gas, a group of elements known for their remarkable stability and low reactivity. This element holds a pivotal position in the table, influencing the chemical properties of its neighboring elements and contributing to the overall structure and organization of the periodic system.
Chemical Properties
Element number 10, with the chemical symbol Ne, possesses an atomic number of 10 and an atomic weight of 20.18. It resides in Group 18 (also known as Group VIIIA) of the periodic table, which comprises the noble gases. These elements are characterized by their complete electron shells, resulting in their lack of reactivity and their existence as monatomic gases under standard conditions.
Position in the Periodic Table
Element number 10 occupies the second period of the periodic table, indicating that its atoms have two energy levels. Within its group, it is the lightest noble gas, followed by helium (He), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn).
This element’s position in the table reflects its unique electronic configuration, which contributes to its distinctive chemical properties and inert nature.
Properties and Characteristics of Element No. 10

Element No. 10, also known as neon, is a noble gas characterized by its unique properties and characteristics. In this section, we will delve into the physical and chemical properties of neon, exploring its reactivity, oxidation states, common isotopes, and diverse applications across various fields.
Physical and Chemical Properties
Neon is a colorless, odorless, and non-flammable gas at room temperature. It has a very low boiling point (-246.08 °C) and a melting point (-248.59 °C), making it one of the coldest elements in the periodic table. Neon is also known for its low reactivity, as it is a noble gas with a full valence electron shell.
This means that it does not readily form chemical bonds with other elements, making it chemically inert.
Reactivity and Oxidation States
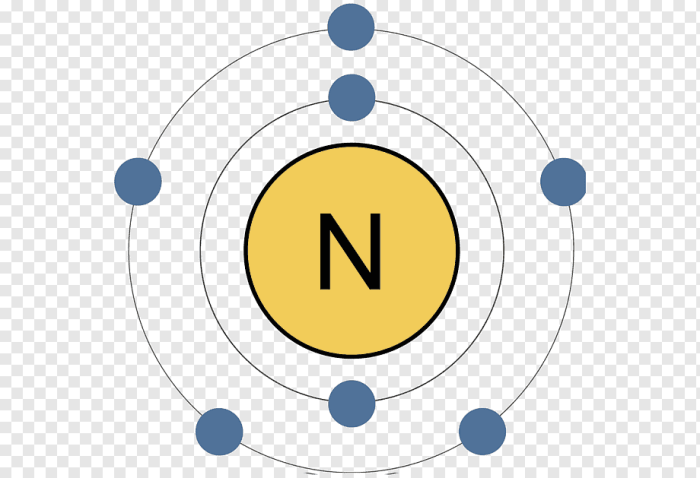
Due to its inert nature, neon does not exhibit variable oxidation states. It typically exists in its elemental form, with a stable electron configuration of 1s 22s 22p 6. This electron configuration gives neon a closed-shell structure, making it unreactive and unlikely to participate in chemical reactions.
Common Isotopes
Neon has three naturally occurring isotopes: neon-20, neon-21, and neon-22. Neon-20 is the most abundant isotope, accounting for approximately 90.48% of natural neon. Neon-21 and neon-22 are less common, with abundances of 0.27% and 9.25%, respectively. These isotopes have similar chemical properties, but their different masses can be used for various applications, such as isotopic labeling in scientific research.
Applications
Despite its low reactivity, neon finds applications in various fields. Its unique properties make it useful in lighting, advertising, and scientific research. For example, neon is used in fluorescent lights, where it emits a characteristic reddish-orange glow when an electric current passes through it.
Neon signs are also popular for advertising due to their bright and attention-grabbing nature.
History and Discovery of Element No. 10

The discovery of element number 10, neon, marked a significant milestone in the field of chemistry. Its journey from obscurity to recognition is a fascinating tale involving meticulous experimentation and scientific breakthroughs.
In 1898, Sir William Ramsay and Morris Travers, two renowned chemists, embarked on a series of experiments to investigate the properties of gases present in the atmosphere. Using a technique known as fractional distillation, they meticulously separated and analyzed various components of air.
Isolation and Identification, Element no 10 crossword clue
During their experiments, Ramsay and Travers observed an unusual gas that exhibited a distinct orange-red glow when subjected to an electrical discharge. This observation hinted at the presence of a new element. To confirm their findings, they repeated their experiments multiple times, carefully isolating and purifying the unknown gas.
Through a series of rigorous tests and measurements, Ramsay and Travers determined the atomic weight and other physical properties of the newly discovered element. They recognized its unique characteristics and realized that it belonged to a previously unknown group of elements, which they termed “noble gases.”
Origin of the Name
The name “neon” was derived from the Greek word “neos,” meaning “new.” This name aptly reflects the element’s novel and unexpected discovery. The designation of element number 10 as “neon” was officially adopted by the International Union of Pure and Applied Chemistry (IUPAC) in 1923, solidifying its place in the periodic table.
Isotopes and Uses of Element No. 10
Element number 10, known as neon, has three naturally occurring isotopes: neon-20, neon-21, and neon-22. Neon-20 is the most abundant isotope, accounting for approximately 90% of the element’s natural abundance. Neon-21 and neon-22 are much less common, with abundances of about 0.27% and 9.23%, respectively.
These isotopes differ in their number of neutrons, which affects their atomic masses and some of their properties. Neon-20 has 10 neutrons, neon-21 has 11 neutrons, and neon-22 has 12 neutrons.
Isotopes and Their Applications
The different isotopes of neon have unique properties that make them suitable for various applications.
- Neon-20:The most abundant isotope of neon is used in a wide range of applications, including lighting, lasers, and cryogenics. It is also used as a tracer gas in leak detection and medical imaging.
- Neon-21:This isotope is used in nuclear magnetic resonance (NMR) spectroscopy, a technique used to study the structure and dynamics of molecules. It is also used in some types of lasers.
- Neon-22:The least abundant isotope of neon is used in some types of lighting and lasers. It is also used as a tracer gas in environmental studies.
Safety Considerations
Neon is a non-toxic gas, but it can be hazardous if inhaled in large quantities. It can cause dizziness, nausea, and vomiting. In extreme cases, it can lead to unconsciousness and even death.
When handling neon, it is important to take precautions to avoid inhaling the gas. This includes using proper ventilation and wearing a respirator if necessary.
Crossword clue “element no 10” may lead you to study chemistry. While you’re at it, why not enhance your knowledge with the oae 043 practice test free ? It can help you ace that upcoming exam. Returning to the crossword, you’ll find that element no 10 is neon.
Element No. 10 in Nature and the Environment: Element No 10 Crossword Clue
Element number 10, neon, is a colorless, odorless, and non-flammable gas that occurs naturally in the Earth’s atmosphere, crust, and oceans.
Natural Occurrence
- Earth’s Atmosphere:Neon is present in the Earth’s atmosphere in trace amounts, making up approximately 0.0018% of its volume.
- Earth’s Crust:Neon is found in minerals such as apatite and zircon, but its concentration is very low, typically less than 1 part per million.
- Oceans:Neon is dissolved in seawater, with a concentration of approximately 0.0002 parts per million.
Geological Processes
Neon plays a minimal role in geological processes due to its low abundance and inert nature. However, it can be used as a tracer gas in studies of groundwater flow and atmospheric circulation.
Environmental Concerns
Neon and its compounds are generally considered to be environmentally benign. However, large-scale industrial processes that involve neon, such as the production of neon signs, can release small amounts of neon into the atmosphere. While neon is not a greenhouse gas, it can contribute to air pollution and potentially affect the ozone layer.
FAQ Explained
What is the chemical symbol for element no. 10?
Ne
What group does element no. 10 belong to in the periodic table?
Group 18 (Noble Gases)
What is a unique application of element no. 10?
Neon lighting, due to its ability to emit a bright red-orange glow when an electric current passes through it.