Chemistry paint by valence electrons takes center stage in this exploration, unraveling the intricate relationship between the fundamental principles of chemistry and the behavior of paint. Valence electrons, the outermost electrons of an atom, play a pivotal role in determining the chemical properties of paint, influencing its adhesion, color, and durability.
Delving into the world of paint chemistry, we will uncover the diverse types of paint based on their valence electron configurations, examining their unique characteristics and properties. We will explore the intricate relationship between valence electrons and paint adhesion, shedding light on the factors that govern the ability of paint to adhere to various surfaces.
Chemistry of Paint by Valence Electrons
The chemical properties of paint are largely determined by the valence electrons of the atoms that make up the paint molecules. Valence electrons are the electrons in the outermost shell of an atom, and they are responsible for the chemical reactions that the atom can undergo.
In the case of paint, the valence electrons of the atoms in the paint molecules interact with the valence electrons of the atoms in the surface being painted. This interaction creates a chemical bond between the paint and the surface, which holds the paint in place.
Role of Valence Electrons in Determining the Chemical Properties of Paint
The number of valence electrons in an atom determines its chemical properties. Atoms with a high number of valence electrons are more reactive than atoms with a low number of valence electrons. This is because atoms with a high number of valence electrons are more likely to lose or gain electrons, which allows them to form chemical bonds with other atoms.
In the case of paint, the number of valence electrons in the atoms of the paint molecules determines the type of chemical bond that the paint will form with the surface being painted. For example, paints that contain atoms with a high number of valence electrons will form covalent bonds with the surface, while paints that contain atoms with a low number of valence electrons will form ionic bonds with the surface.
Examples of How Valence Electrons Influence the Behavior of Paint
The valence electrons of the atoms in paint molecules also influence the behavior of the paint. For example, the valence electrons of the atoms in the paint molecules determine the color of the paint. The color of a paint is determined by the wavelength of light that is absorbed by the paint.
The wavelength of light that is absorbed by a paint is determined by the energy of the valence electrons in the atoms of the paint molecules.
The valence electrons of the atoms in paint molecules also determine the durability of the paint. The durability of a paint is determined by its resistance to wear and tear. The resistance of a paint to wear and tear is determined by the strength of the chemical bonds between the paint and the surface being painted.
The strength of the chemical bonds between the paint and the surface being painted is determined by the number of valence electrons in the atoms of the paint molecules.
Types of Paint Based on Valence Electrons
The valence electrons of an atom play a crucial role in determining its chemical properties, including its reactivity and ability to form bonds. In the context of paint, the valence electrons of the pigments and binders influence the paint’s characteristics, such as color, durability, and adhesion.
Based on the valence electron configurations of their components, paints can be broadly classified into two main types:
Ionic Paints
Ionic paints are composed of pigments and binders that contain ions, which are atoms or molecules that have gained or lost electrons. The valence electrons of the ions are involved in the formation of ionic bonds, which are strong electrostatic attractions between oppositely charged ions.
Ionic paints are characterized by their high durability and resistance to fading. They are commonly used in industrial and commercial applications, such as on bridges and buildings, where longevity and resistance to harsh environmental conditions are essential.
Examples of ionic paints include:
- Titanium dioxide (TiO2): A white pigment with a valence electron configuration of 3d2 4s2.
- Iron oxide (Fe2O3): A red or brown pigment with a valence electron configuration of 3d6 4s2.
- Zinc oxide (ZnO): A white pigment with a valence electron configuration of 3d10 4s2.
Covalent Paints
Covalent paints are composed of pigments and binders that contain covalent bonds, which are formed by the sharing of electrons between atoms. The valence electrons of the atoms involved in the covalent bonds are responsible for holding the atoms together.
Covalent paints are characterized by their vibrant colors and flexibility. They are commonly used in artistic and decorative applications, such as in paintings and murals, where aesthetics and ease of application are important.
Examples of covalent paints include:
- Phthalocyanine blue: A blue pigment with a valence electron configuration of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 5d10 6s2 6p6 4f14 5f14 6f2.
- Cadmium yellow: A yellow pigment with a valence electron configuration of 4d10 5s2.
- Alizarin crimson: A red pigment with a valence electron configuration of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2 4d10 5p6.
Valence Electrons and Paint Adhesion
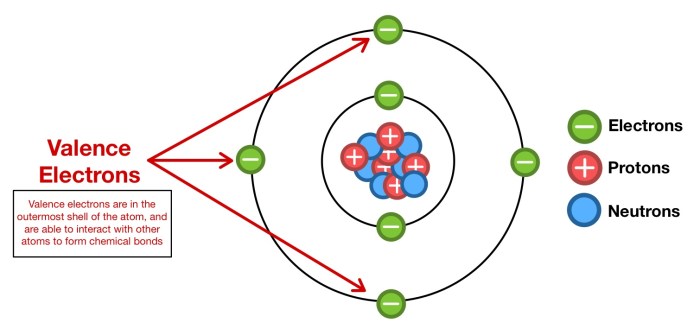
Valence electrons play a crucial role in determining the adhesion properties of paint. Adhesion refers to the ability of paint to stick to a surface and resist peeling or flaking. The adhesion of paint is influenced by various factors, including the number of valence electrons in the paint molecules, the type of surface being painted, and the presence of any contaminants or impurities.
Factors Influencing Paint Adhesion
The number of valence electrons in the paint molecules affects the strength of the intermolecular forces between the paint and the surface. Stronger intermolecular forces result in better adhesion. For example, paints containing molecules with a higher number of valence electrons, such as acrylic paints, typically exhibit better adhesion than paints with molecules containing a lower number of valence electrons, such as alkyd paints.
The type of surface being painted also influences paint adhesion. Different surfaces have different chemical compositions and surface energies, which affect the strength of the intermolecular forces between the paint and the surface. For example, paint adheres better to rough surfaces than smooth surfaces because the increased surface area provides more opportunities for intermolecular interactions.
The presence of any contaminants or impurities on the surface can also affect paint adhesion. Contaminants and impurities can interfere with the formation of intermolecular forces between the paint and the surface, resulting in poor adhesion. Therefore, it is important to clean and prepare the surface properly before painting to ensure good adhesion.
Manipulating Valence Electrons to Improve Paint Adhesion
The adhesion of paint can be improved by manipulating the valence electrons in the paint molecules. One way to do this is by adding additives to the paint that contain molecules with a high number of valence electrons. These additives can increase the overall number of valence electrons in the paint, resulting in stronger intermolecular forces and better adhesion.
Another way to improve adhesion is by using primers. Primers are applied to the surface before painting and contain molecules that form strong bonds with both the surface and the paint, creating a stronger bond between the two.
Valence Electrons and Paint Color
Valence electrons play a crucial role in determining the color of paint. The number and arrangement of valence electrons in an atom or molecule influence the absorption and reflection of light, which in turn determines the perceived color.
Different Valence Electron Configurations and Color
The energy levels of valence electrons dictate the wavelengths of light that are absorbed or reflected. When white light strikes a surface, some wavelengths are absorbed and others are reflected. The reflected wavelengths correspond to the colors we perceive.For example, a substance with valence electrons in a low-energy level will absorb high-energy, short-wavelength light, such as blue or violet.
The reflected light will be in the longer wavelength range, such as red or orange. This is why many metal oxides, such as iron oxide (rust), appear red or orange.
Manipulation of Valence Electrons for Color Creation
The valence electron configuration of a substance can be manipulated to create specific colors of paint. This is achieved through chemical reactions or the addition of pigments.One common method is to use dyes or pigments that contain conjugated double bonds.
These bonds allow electrons to move freely along the molecule, creating a wider range of energy levels. This results in the absorption of a broader spectrum of light and the reflection of a more specific color.Another approach is to use transition metal ions as pigments.
These ions have partially filled d orbitals, which can interact with light in a way that produces specific colors. For example, cobalt ions produce blue pigments, while copper ions produce green pigments.
Valence Electrons and Paint Durability
The durability of paint is influenced by the number of valence electrons present in the atoms of the pigments and binders used in the paint formulation. Valence electrons are the electrons in the outermost shell of an atom, and they determine the chemical properties of the atom.
The more valence electrons an atom has, the more reactive it is. This reactivity affects the durability of paint because it determines how easily the paint can be oxidized or degraded by exposure to UV light and weathering.
Factors Influencing Paint Durability
- Exposure to UV light:UV light can break down the chemical bonds in paint, causing it to fade and become brittle. Paints with more valence electrons are more resistant to UV damage because the valence electrons help to protect the chemical bonds from breaking.
- Weathering:Weathering is the process of paint being exposed to the elements, such as rain, snow, and wind. Weathering can cause paint to fade, chip, and peel. Paints with more valence electrons are more resistant to weathering because the valence electrons help to keep the paint film intact.
- Type of binder:The binder is the component of paint that holds the pigments together. The type of binder used in a paint can affect the durability of the paint. Binders with more valence electrons are more resistant to degradation, which can help to extend the life of the paint.
Examples of Valence Electrons and Paint Durability, Chemistry paint by valence electrons
- Titanium dioxide (TiO2)is a common white pigment used in paint. TiO2 has a high number of valence electrons, which makes it very resistant to UV damage and weathering. This is why TiO2 is often used in exterior paints that are exposed to the elements.
- Carbon black (C)is a common black pigment used in paint. C has a low number of valence electrons, which makes it less resistant to UV damage and weathering. This is why C is often used in interior paints that are not exposed to the elements.
Valence Electrons and Paint Applications
Valence electrons play a crucial role in determining the properties of paint, influencing its suitability for various applications. By understanding the valence electron configurations of different pigments and binders, it becomes possible to tailor the paint’s characteristics to meet specific performance requirements.
Paints for Interior and Exterior Applications
- Paints for interior applications are typically formulated with pigments that have valence electrons that form strong covalent bonds with the binder. This results in paints with high durability and resistance to fading, making them suitable for use in areas with moderate wear and tear.
- Paints for exterior applications require pigments with valence electrons that can form stable bonds with the binder and resist degradation from environmental factors such as UV radiation and moisture. These paints are often formulated with inorganic pigments, such as metal oxides, which have valence electrons that form strong ionic bonds.
Paints for Different Surfaces
- Paints designed for metal surfaces typically contain pigments with valence electrons that can form strong bonds with the metal substrate. These bonds prevent corrosion and ensure the paint’s adhesion and durability.
- Paints for wooden surfaces often utilize pigments with valence electrons that can form hydrogen bonds with the cellulose fibers in the wood. This results in paints that penetrate the wood’s surface, providing excellent adhesion and protection.
Paints for Special Applications
- Conductive paints contain pigments with valence electrons that can form delocalized electron systems. These paints are used in electronic applications, such as printed circuit boards, due to their ability to conduct electricity.
- Fire-retardant paints incorporate pigments with valence electrons that can form stable bonds with oxygen, preventing the spread of flames. These paints are used in areas where fire safety is a concern, such as public buildings and industrial facilities.
Top FAQs: Chemistry Paint By Valence Electrons
What is the role of valence electrons in paint chemistry?
Valence electrons play a crucial role in determining the chemical properties of paint, influencing its adhesion, color, and durability.
How do valence electrons affect paint adhesion?
Valence electrons influence the adhesion properties of paint by interacting with the surface of the substrate, forming chemical bonds that hold the paint in place.
Can valence electrons be manipulated to improve paint durability?
Yes, by understanding the relationship between valence electrons and durability, we can manipulate these electrons to enhance the paint’s resistance to UV light and weathering.