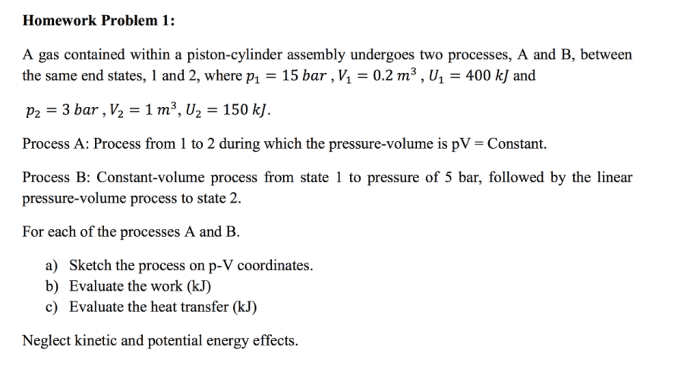
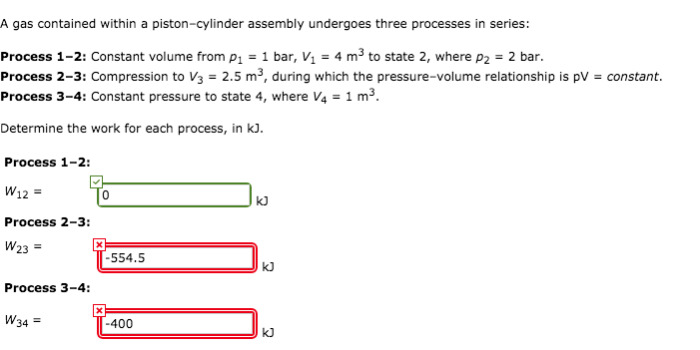
A gas contained within a piston-cylinder assembly undergoes two processes, providing a unique opportunity to explore the intricate relationship between pressure, volume, and temperature. This exploration delves into the fundamental principles of thermodynamics, examining how gases behave under varying conditions.
The journey begins with an examination of the two thermodynamic processes, uncovering their distinct characteristics and the resulting changes in pressure, volume, and temperature. The spotlight then shifts to the gas itself, identifying its type and properties, and tracing its behavior throughout the processes.
Thermodynamic Processes: A Gas Contained Within A Piston-cylinder Assembly Undergoes Two Processes
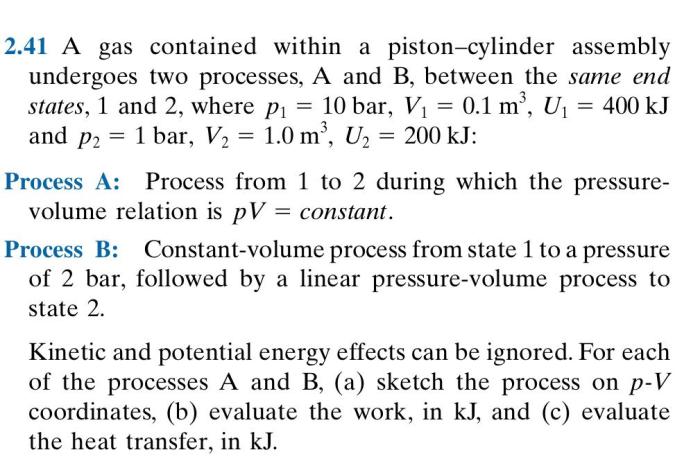
The gas within the piston-cylinder assembly undergoes two thermodynamic processes: an isothermal process and an adiabatic process.
During the isothermal process, the temperature of the gas remains constant. The process is characterized by an increase in volume and a decrease in pressure. The work done by the gas is equal to the area under the curve on a pressure-volume diagram.
During the adiabatic process, there is no heat transfer between the gas and its surroundings. The process is characterized by an increase in pressure and temperature and a decrease in volume. The work done by the gas is equal to the change in internal energy.
Gas Behavior
The gas involved in the processes is an ideal gas. An ideal gas is a theoretical gas that obeys the ideal gas law, which states that the pressure, volume, and temperature of a gas are related by the equation PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature.
During the isothermal process, the gas behaves as an ideal gas. The pressure and volume change in accordance with the ideal gas law. During the adiabatic process, the gas does not behave as an ideal gas. The pressure and volume change in a way that is not described by the ideal gas law.
Work and Heat Transfer, A gas contained within a piston-cylinder assembly undergoes two processes
The work done by the gas during the isothermal process is equal to the area under the curve on a pressure-volume diagram. The heat transfer between the gas and its surroundings during the isothermal process is equal to the work done by the gas.
The work done by the gas during the adiabatic process is equal to the change in internal energy of the gas. The heat transfer between the gas and its surroundings during the adiabatic process is zero.
Energy Balance
An energy balance equation for the gas during each process can be written as follows:
Isothermal process: Q – W = 0
Adiabatic process: Q = 0
where Q is the heat transfer, W is the work done, and U is the internal energy of the gas.
The energy balance equation can be used to determine the properties of the gas. For example, the internal energy of the gas can be determined by measuring the heat transfer and work done during a process.
Graphical Representation
A pressure-volume (P-V) diagram can be used to visualize the behavior of the gas during the two processes.
The P-V diagram for the isothermal process is a rectangular hyperbola. The area under the curve on the P-V diagram is equal to the work done by the gas.
The P-V diagram for the adiabatic process is a curve that is steeper than the rectangular hyperbola for the isothermal process. The area under the curve on the P-V diagram is equal to the change in internal energy of the gas.
Answers to Common Questions
What are the two thermodynamic processes that the gas undergoes?
The gas undergoes an isothermal process and an adiabatic process.
How does the gas’s behavior change during the two processes?
During the isothermal process, the gas’s temperature remains constant, while its volume and pressure change. During the adiabatic process, the gas’s temperature and pressure change, while its volume remains constant.